Abstract
Background. Oxidative stress contributes to the pathophysiology of sickle cell anemia (SCA). Oral l-glutamine increases the proportion of reduced NAD in sickle erythrocytes,1 which may decrease oxidative stress and SCA complications.2 Recently, a multicenter, randomized, placebo-controlled, double-blind, phase 3 trial of l-glutamine in SCA reported that the l-glutamine group had fewer pain crises and hospitalizations over 48 weeks of treatment than the placebo arm.3 There was a consistent treatment effect of l-glutamine in subjects who used and did not use hydroxyurea. The purpose of the present analysis was to determine if there was consistent use of hydroxyurea in both arms of the trial and if l-glutamine therapy led to a reduction in hemolysis and an amelioration of anemia.
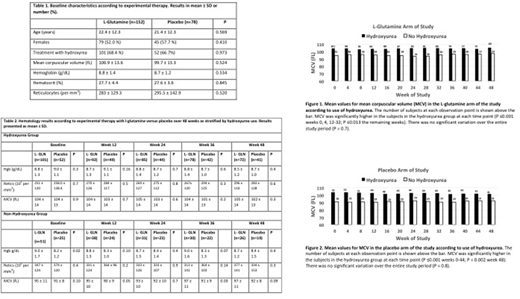
Methods. Because increase in mean corpuscular volume (MCV) is a sensitive indicator of hydroxyurea therapy,4 we used MCV as a surrogate marker of hydroxyurea compliance. We analyzed MCV at 4-week intervals according to hydroxyurea therapy separately in the l-glutamine and placebo arms. We also analyzed hemoglobin concentration and reticulocyte count at 12-week intervals according to experimental treatment with l-glutamine versus placebo as stratified by hydroxyurea use. We imputed missing variables by carrying the last measured value forward. We used the student t-test to compare linear variables, Pearson chi square to compare categorical variables and repeated measures analysis of variance to analyze trends over time.
Results. The patients receiving l-glutamine and placebo were well matched (Table 1). During the 48-week trial, the MCV was consistently greater in patients using hydroxyurea than those not using hydroxyurea in both the l-glutamine arm (Figure 1) and the placebo arm (Figure 2). Furthermore, there was no significant change over 48 weeks in the MCV in the patients using hydroxyurea in either arm (P >0.7). In the hydroxyurea group, there were no significant differences according to experimental therapy with l-glutamine versus placebo in hemoglobin concentration or reticulocyte count at 12 weekly intervals. In the group not using hydroxyurea, the hemoglobin concentration was significantly higher at baseline in patients given l-glutamine compared to those given placebo, but this observation did not persist at the subsequent determinations; reticulocyte counts did not differ at any time point (Table 2). We performed many multivariate and repeated measures analyses of hemoglobin concentration and reticulocyte count that adjusted for age, gender and blood transfusions, but did not observe evidence that l-glutamine therapy was associated with an increase in hemoglobin concentration and/or a decline in reticulocyte count.
Discussion. Based on the MCV, patients who were assigned to hydroxurea complied with hydroxyurea therapy at baseline and throughout the study; this supports the concept that the beneficial clinical effects of l-glutamine therapy for SCA are additive to those of hydroxyurea. Therapy with l-glutamine did not reduce hemolysis or improve anemia, which parallels observations with therapy with an antibody against the adhesion molecule, P-selectin.5 The mechanism whereby l-glutamine leads to reduced vaso-occlusive complications in SCA requires further investigation. Glutamine levels are lower in sickle erythrocytes compared to erythrocytes from healthy volunteers.6 L-Glutamine reduces intercellular adhesion molecule expression in endothelial cells,7 decreases adhesion of sickle erythrocytes to endothelial cells,8 attenuates the expression of endothelial adhesion molecules and T cell migration markers when the intestines are injured, 9,10 and inhibits IL-6 and TNF-α production in LPS-stimuated monocytes.11 Future research will need to assess the effect of l-glutamine on redox potential, adhesion molecule expression, and inflammatory marker expression in erythrocytes, other circulating blood cells and the vascular endothelium.
References
Am J Hematol. 1998;58(2):117-121.
IUBMB Life. 2012;64(1):72-80.
N Engl J Med. 2018;379(3):226-235.
Medicine (Baltimore). 1996;75(6):300-326.
N Engl J Med. 2017;376(5):429-439.
Blood. 2008;111(1):402-410.
J Reprod Med. 2006;51(3):193-198.
BMC Blood Disord. 2005;5:4.
Eur J Pharmacol. 2010;643(2-3):304-315.
Mediators Inflamm. 2014;2014:837107.
Cytokine. 2013;62(1):52-57.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.